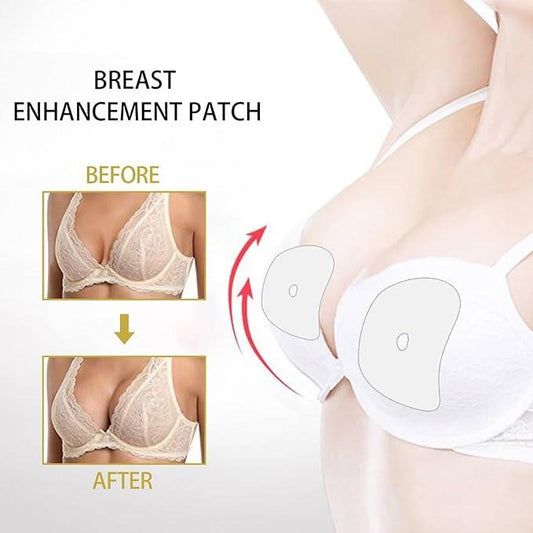
Evebra & Cancer Safety
Share
Mechanical Tissue Expansion & Cancer: Understanding the Science and Safety of EVE in Breast Reconstruction
Introduction to Mechanical Tissue Expansion: A Foundation of Reconstructive Medicine
Mechanical forces stimulating tissue growth have long served as a cornerstone technique in reconstructive surgery. This fundamental principle – harnessing the body’s innate ability to generate new tissue in response to sustained mechanical forces – has revolutionized how a surgeon approaches complex repairs. EVE utilizes precisely controlled, gentle mechanical tension, completely devoid of electromagnetic, ultrasonic, or ionizing radiation energy, to stimulate natural tissue growth, offering a different path for patients undergoing certain reconstruction journeys.
The concept of using controlled expansion has profoundly impacted surgery, offering a remarkable pathway for the body to effectively “grow” additional skin and essential soft tissue. This tissue expansion process can eliminate the need for extensive skin grafting from distant body sites (like the lower abdomen in some flap procedures), which carries its own risk factors and complications, including donor site morbidity and potential mismatches in tissue quality or color.
In breast reconstruction surgery, particularly following mastectomy, the use of acellular dermal matrix as a supportive material is a common practice during the procedure to enhance the placement of implants and internal tissue expanders. This mesh, derived from processed donor skin, aims to minimize infection and rejection risks, providing reliable scaffolding during this type of reconstruction.
Whether the goal is repairing defects resulting from congenital conditions, severe trauma, burns, or post-cancer surgery (like mastectomy), mechanical methods provide a natural, biocompatible way to regenerate the necessary tissue volume in situ. As technological advancements like EVE simplify and optimize this approach, rigorously evaluating their long-term safety remains paramount, especially compared to traditional surgery involving implants.
What is EVE and How Does It Work? The Power of Gentle Tension
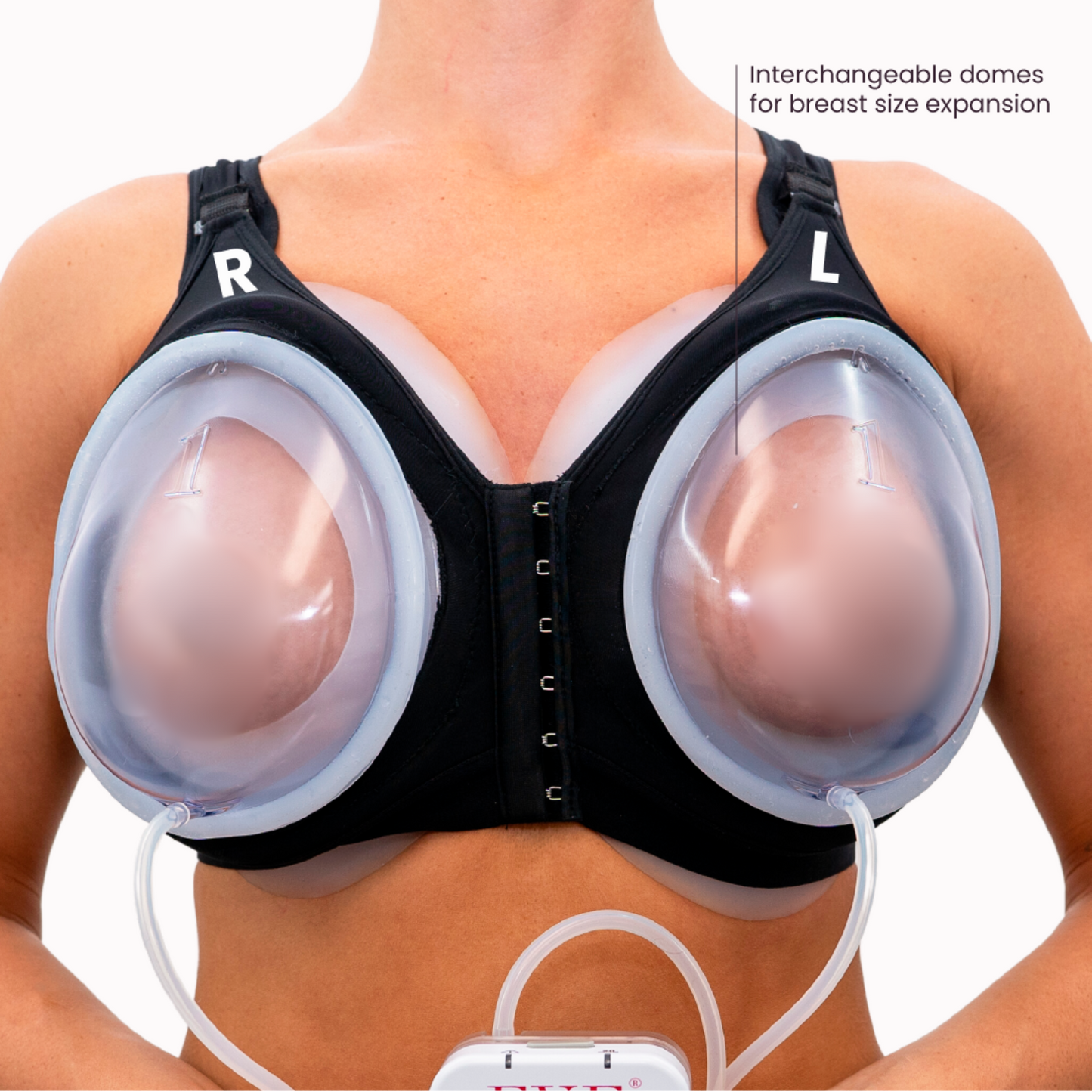
EVE (External Vacuum Expander) represents a sophisticated evolution in external methods for encouraging tissue growth. It achieves controlled growth through sustained, gentle mechanical tension, operating on similar biological principles as the internal tissue expander used in conventional breast reconstruction, but without invasive surgery. By applying mild, continuous negative pressure (vacuum) within specially designed domes placed over the breast area, EVE creates a gentle, outward pull on the breast skin and underlying structures. This sustained tension signals the normal tissues to adapt and grow via mechanotransduction, a natural adaptive and healing process fundamental to wound healing.
The core principle is simple: gradually stretching the skin and underlying soft tissues stimulates resident cells to divide and increase extracellular matrix production, leading to a net increase in tissue volume and potentially affecting breast size. EVE's external design allows individuals to harness benefits like creating well-matched, sensate tissue without the surgical procedures, and risks associated with the surgeon places internal expanders and eventual implants.
Crucially, EVE operates purely through mechanical force, using no electromagnetic, thermal, or ionizing radiation energy. This distinction is vital, alleviating concerns about energy-induced tissue damage, DNA mutations, or theoretical oncogenesis sometimes associated with other energy modalities. Its safety profile is anchored in its purely mechanical action, a key difference from some other medical treatments.
Navigating the EVE Experience: Practical Aspects and Support
Understanding the science and safety of EVE is crucial, but prospective users often have practical questions about the day-to-day procedure and what the treatment entails. Using an external vacuum expander like EVE requires commitment and adherence to a specific protocol prescribed by the supervising doctor or surgeon. Typically, this involves wearing the device for several hours each day, often overnight, for a period of several months. While the design emphasizes comfort, some users may initially experience minor complications like skin redness or irritation where the domes seal against the body. Proper fitting and skin care are essential to mitigate these issues, and guidance from the healthcare team is invaluable here. Adjusting to sleeping with the device also requires a period of adaptation for many patients.
The healthcare team, including the primary surgeon or doctor overseeing the reconstruction or enhancement journey, plays a vital role beyond the initial consultation. Immediate breast reconstruction often involves the use of tissue expanders placed under the skin at the same time as a mastectomy. Regular follow-up appointments are necessary to monitor progress, address any concerns or side effects, and ensure the device is functioning correctly. This ongoing support network is critical for patient success and managing expectations throughout the expansion period. They can offer practical tips, troubleshoot issues, and provide encouragement, acknowledging the dedication required. Open communication about any discomfort or pain experienced allows for timely adjustments to the treatment plan, ensuring the procedure remains as comfortable and effective as possible for the individual undergoing breast reconstruction or enhancement.
Managing daily life while adhering to the EVE protocol involves planning. While the device is often worn during sleep, integrating wear time around work, family, and social commitments requires consideration. Many patients find establishing a consistent routine helps immensely. Although strenuous upper body exercise might be restricted while wearing the domes, most normal activities can typically be maintained. Discussing lifestyle factors with the doctor ensures personalized advice. Furthermore, understanding that results accumulate gradually over weeks and months is key; this isn’t an instant transformation but a physiological process leveraging the body’s natural ability to generate new tissue when appropriately stimulated by the expander.
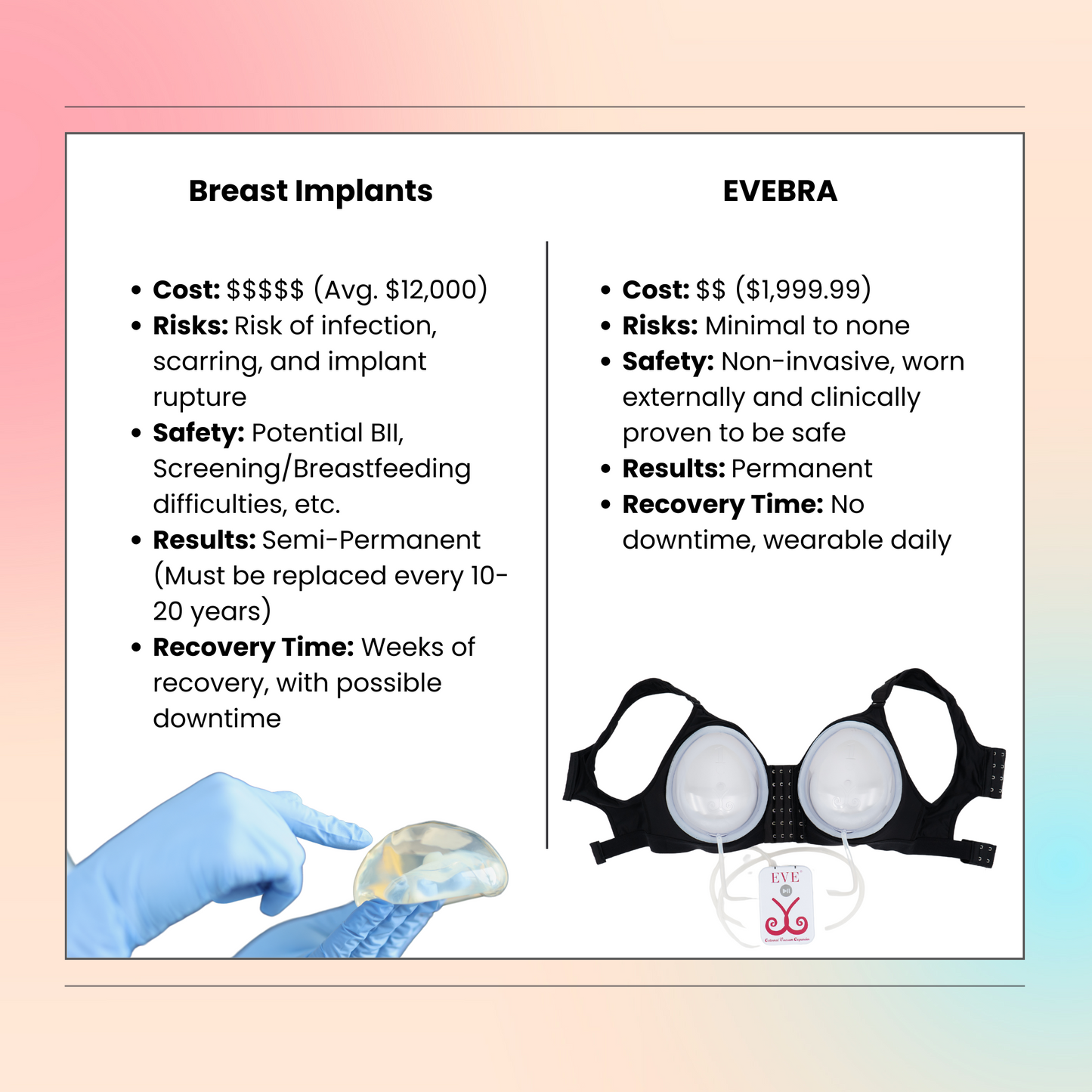
Compared to surgical breast reconstruction surgery involving internal tissue expanders and subsequent breast implant placement, the EVE procedure sidesteps immediate surgical risks like anesthesia reactions, significant pain, bleeding, or deep infection. However, the trade-off is the extended period of device wear and the potential for surface-level skin irritation. Choosing between these reconstruction pathways often involves weighing the desire to avoid invasive surgery and implants against the time commitment and compliance needed for external expansion. Each patient’s anatomy, lifestyle, desired outcome for their reconstructed breast, and tolerance for different types of treatment burden influence this decision, best made after thorough discussion with their surgeon.
Cellular Mechanisms: How Mechanotransduction Drives Healthy Growth
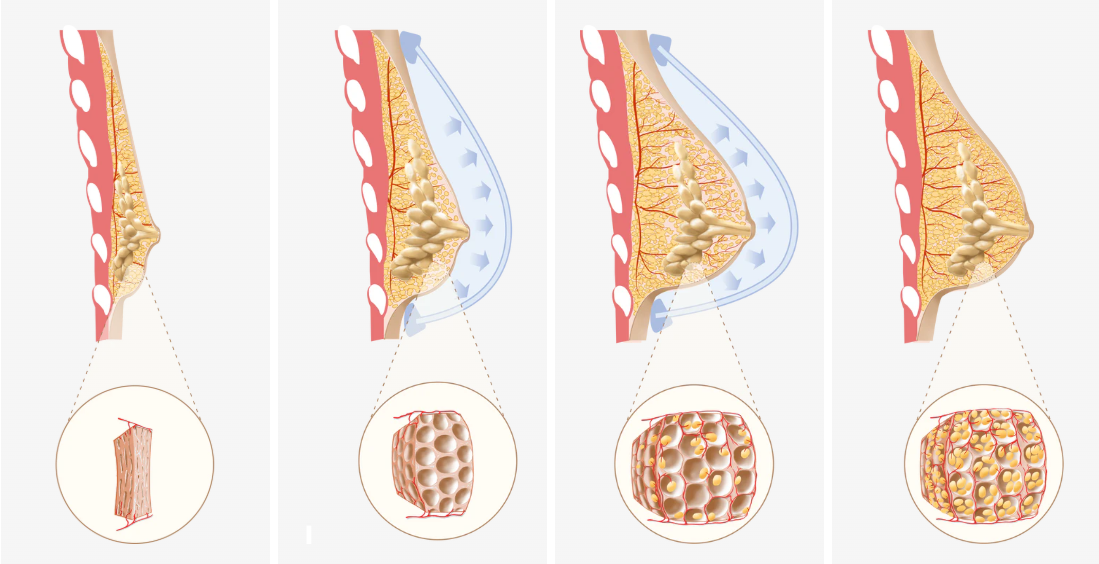
The process enabling this method lies in mechanotransduction: how living cells sense physical forces and translate them into biochemical signals dictating behaviour. When EVE applies gentle, sustained tension, specialized receptors (like Integrins linking the cytoskeleton to the external extracellular matrix - ECM) and the internal cytoskeleton detect this strain. Stretch-Activated Ion Channels may open, allowing ions like calcium to flow, triggering cellular responses like proliferation. The Cytoskeleton actively transmits forces, influencing gene expression.
These events activate pathways (MAPK/ERK, PI3K/Akt), stimulating healthy, adaptive responses under controlled tension:
1. Cell Proliferation: Controlled cell division increases numbers.
2. ECM Production: Fibroblasts boost collagen/elastin synthesis, expanding the tissue matrix.
3. Angiogenesis: Formation of new blood vessels supports the growing tissue mass, ensuring adequate blood supply and oxygen. Good vascularity from blood vessels is key.
4. Tissue Remodeling: Architecture adapts to increased volume, maintaining function and potentially improving nerve supply integration over time compared to some alternatives.
Mechanical Forces: Non-Carcinogenic by Nature – A Deep Dive into the Evidence
Long-term safety, particularly concerning breast cancer risk, is fundamental. Cancer biology establishes that cancer initiation requires genetic mutations or epigenetic alterations, often caused by carcinogens (radiation, chemicals, chronic inflammation). Mechanical forces, like EVE's tension, are not mutagenic. They don't damage DNA or cause the genetic changes underpinning cancer development. Peer reviewed studies support this.
Examining cellular responses to controlled mechanical tension reveals pathways linked to normal tissue remodeling and wound healing. Cells respond appropriately by reinforcing structure, stimulating collagen production, and organizing the ECM – none intrinsically linked to carcinogenesis. Controlled mechanical stimulation might even help maintain tissue homeostasis.
Cancer Biology Fundamentals: Why Mechanical Force Isn't a Trigger
Understanding cancer biology clarifies why this mechanical approach is safe. Cancer is uncontrolled growth driven by genetic aberrations affecting cell division, death, and spread (hallmarks of cancer). These mutations often arise from exposure to carcinogens or DNA replication errors.
Mechanical forces from EVE lack the energy or reactivity to cause these initiating mutations. Gentle stretching activates physiological growth pathways within the body's normal regulatory framework, encouraging healthy cells to multiply. It doesn't provide the mutagenic spark for cancer or typically create chronic inflammation sometimes linked to cancer surgery recovery complications. The procedure aims to mimic physiological growth.

Comparing EVE's Mechanical Effect to Natural Gravity: An Intuitive Safety Benchmark
Consider gravity's constant mechanical tension on large, natural breasts. This represents lifelong exposure to significant mechanical stress. Extensive epidemiological studies show women with naturally larger breasts have no increased breast cancer risk. Some studies suggest a potentially lower incidence per gram of tissue.
This natural experiment provides a powerful analogy. If lifelong gravity-induced tension doesn't increase cancer risk, the temporary, controlled forces from devices like EVE over months should also be safe. This comparison grounds the safety assessment in real-world physiology, relevant for anyone considering options affecting the breast area.
Evidence Spotlight: Decades of Safety in Post-Mastectomy Breast Reconstruction
The most compelling clinical evidence for the safety of mechanical forces comes from decades using internal tissue expanders in breast reconstruction following mastectomy for breast cancer. In this common reconstructive surgery procedure, the surgeon places a temporary internal expander under the remaining skin and muscle of the chest wall. This tissue expander is gradually filled with saline (salt water) over weeks or months, stretching the tissues to create a pocket, ideally with enough skin for a permanent breast implant or autologous tissue reconstruction.
The frequency of these internal expander inflations typically involves weekly visits to the doctor or surgeon, where patients may experience tightness and pain. The role of the tissue expander is crucial in creating the necessary space and breast mound foundation for permanent implants. This internal expansion process involves significant mechanical tension, similar in principle to EVE's external tension.
This procedure is performed on patients who have had breast cancer, where microscopic residual tissue may remain post-mastectomy. Despite this, numerous large-scale studies and extensive clinical experience show no increased risk of local cancer recurrence attributable to the expansion itself. Leading plastic surgery societies endorse this treatment as a standard, safe component of breast reconstruction. Meta-analyses confirm oncologic safety across different groups, including those receiving adjuvant therapies like chemotherapy or radiation therapy. The absence of signals suggesting mechanical forces "wake up" dormant cancer cells in this context provides reassurance about using these forces, whether via internal tissue expanders and subsequent implants or external devices like EVE. This helps address higher risk concerns.
Skin Expansion and Cancer Incidence: Lessons from the Body's Largest Organ
Skin, the body's largest organ and prone to cancer (primarily UV-induced), has undergone millions of successful therapeutic tissue generation procedures worldwide for reconstruction. Surgeons have been expanding skin for decades.
Throughout this history, no credible reports link cancer development within the expanded skin to the expansion itself. This vast "natural experiment" highlights the non-carcinogenic nature of controlled mechanical methods. Studies show expanded skin maintains normal architecture without precancerous/cancerous changes. If mechanical methods were carcinogenic, evidence would exist; its absence is key.
Natural Body Growth Driven by Mechanical Forces: Physiology's Endorsement
Natural growth involves mechanical forces. Bones remodel under load; muscle grows with exercise stress; skin stretches safely during growth spurts/pregnancy. Organs adapt under mechanical force. These examples show the body's ability to safely adapt to mechanical stress. Therapeutic methods like EVE leverage these safe pathways, co-opting a natural mechanism.
Scientific and Epidemiological Evidence: A Consensus on Safety
Broader scientific literature and epidemiological data support the safety conclusion. Basic science illuminates healthy mechanotransduction pathways. Large-scale studies show no increased cancer risk from these procedures. Major reconstruction centers routinely use mechanical methods. Longitudinal studies tracking outcomes confirm the absence of unusual cancer risks. Lack of credible evidence linking devices like EVE to cancer provides strong confidence for the healthcare team, including the doctor and surgeon, recommending these therapies.
The Role of Vacuum in Tissue Expansion: Ensuring Gentle, Uniform Tension
EVE's vacuum (negative pressure) is key:
* Consistent Tension: Uniform pull promotes even growth across the breast skin.
* Low Magnitude Force: Controlled vacuum provides sufficient stimulus without causing tissue damage.
* Non-Invasive Application: Force applied externally.
* Safety: Purely mechanical force generator, no harmful energy.
This controlled vacuum stimulates cellular proliferation, ECM deposition, and angiogenesis (new blood vessels formation, enhancing blood supply). Regular use yields a gradual, natural increase in tissue volume. The vacuum method ensures tension is applied safely for desired tissue generation.
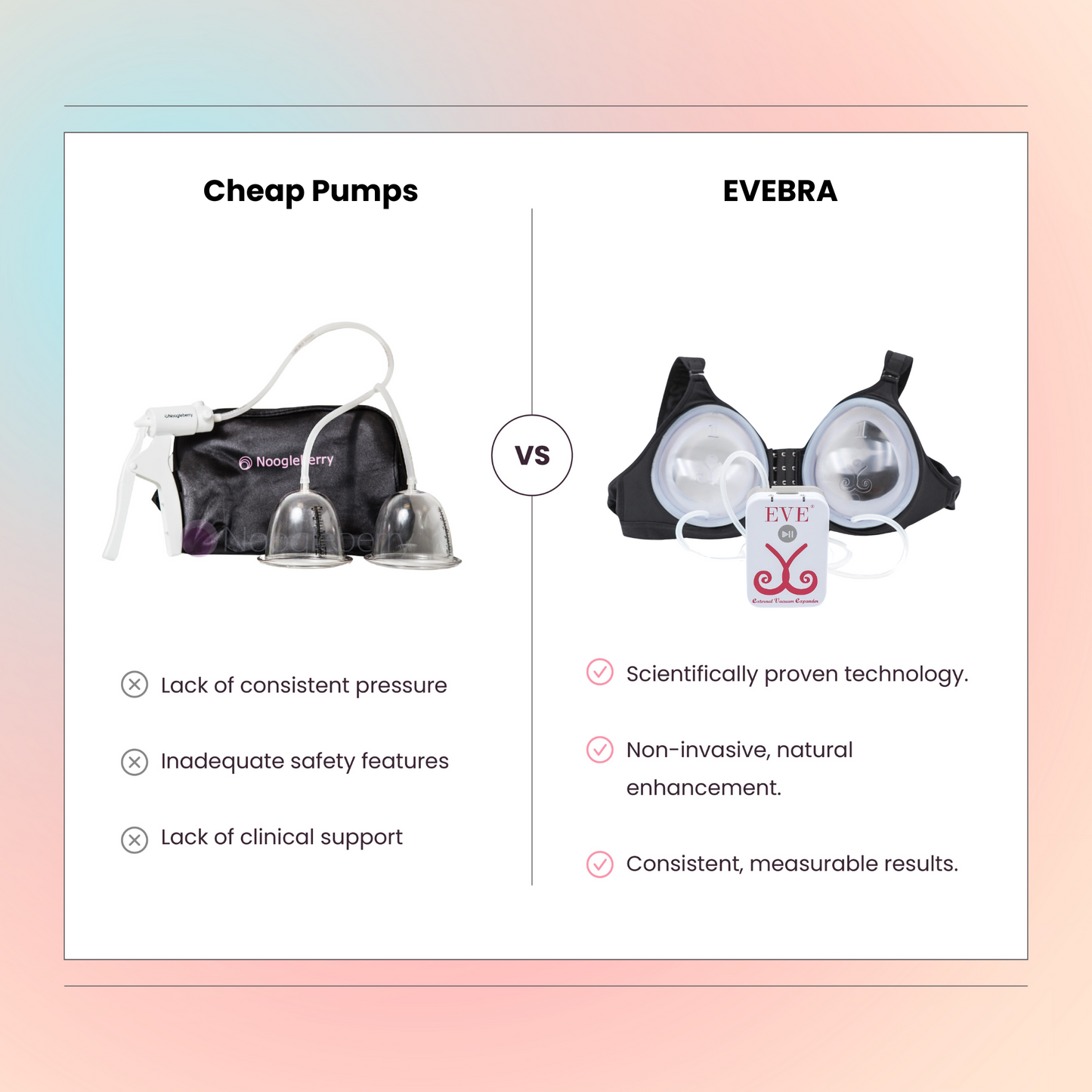
Brava vs. EVE: A Historical Perspective and Technological Evolution
External vacuum-assisted methods for breast enhancement and reconstruction gained prominence with Brava, developed by Dr. Roger Khouri (et al. implicitly referenced). Clinical studies showed its ability to increase volume. However, original systems often required long daily wear, causing compliance and comfort issues.
EVE evolved from Brava's principles, incorporating advancements:
* Enhanced Comfort: Softer, biocompatible materials minimize irritation.
* Optimized Pressure Profiles: Refined vacuum levels maximize stimulation, minimize discomfort.
* Improved Ease of Use: Easier application, removal, maintenance.
* Potentially Shorter Wear Times: Aiming for efficacy with adjusted schedules.
EVE builds on Brava's validated foundation (supported by multiple peer reviewed studies), refining the technology for better tolerance and satisfaction, making it a practical non-invasive option for breast reconstruction or enhancement.
Regulatory Landscape and Clinical Validation of External Tissue Expanders
Medical devices like EVE undergo regulatory oversight (e.g., FDA 510(k) clearance). This signifies the device is "substantially equivalent" to a predicate device in intended use, safety, and effectiveness. The review assesses design, materials, performance, and labeling against safety standards. FDA review includes potential risks like biocompatibility. The history of clearance underscores acceptance of the safety profile.
External vacuum expansion efficacy and safety are documented in numerous peer-reviewed studies, often published in plastic surgery journals (e.g., work by Khouri et al.). These provide data on volume gains, outcomes, and adverse events, consistently showing low rates of serious complications and reinforcing the safety profile.
Addressing Myths and Common Questions About Mechanical Expansion and Cancer
Despite scientific consensus, myths arise:
* Myth: Stretching 'activates' dormant cancer cells. Fact: No credible evidence. Recurrence drivers are distinct from mechanical force. Post-mastectomy reconstruction data refutes this.
* Myth: Vacuum pulls toxins. Fact: Body's filtration systems aren't overridden; force is superficial, primarily affecting skin and subcutaneous fat.
* Myth: Any growth process increases risk. Fact: Cancer is uncontrolled growth via mutation. This method stimulates controlled, physiological growth.
* Question: Safe for high-risk (BRCA) women? Fact: Mechanism isn't mutagenic. Requires personalized discussion with oncologist/genetic counselor/ doctor. EVE's mechanism (mechanical force) differs from known risk factors in BRCA carriers (hormones, radiation). The decision depends on the overall clinical picture and treatment plan.
Physiological mechanical forces foster healthy, organized responses, unlike chaotic, mutation-driven cancer.
Understanding Risks and Complications in Context
While EVE avoids surgical complications, understanding potential issues is important. For internal tissue expander based breast reconstruction surgery, complications can include infection, implant rupture (a small risk with any breast implant, including saline implants), excessive scar tissue formation (capsular contracture), pain, and issues related to the chest wall or overlying muscle. A rare type of lymphoma (BIA-ALCL) has been associated with certain textured implants. Managing these requires close work with the surgeon and healthcare team. If an infection occurs, treatment may be needed until the infection clears. EVE's complications are typically minor skin irritation, but proper use guided by the doctor is crucial. Discussing all potential risk factors and complications for any chosen reconstruction procedure is vital.
Long-Term Perspectives: Tissue Quality and Psychological Well-being
The decision-making process for breast reconstruction after mastectomy is complex, involving numerous risk factors and considerations beyond just the procedure itself. Factors like the stage of breast cancer, prior treatments (like radiation therapy), overall health, body type, available donor sites for autologous tissue (like the lower abdomen), and personal preferences regarding surgery, implants, and recovery time must all be weighed. The surgeon plays a crucial role in explaining all viable options, including immediate breast reconstruction versus delayed, EVE, implant-based reconstruction (often involving a tissue expander initially), autologous flaps, or choosing no reconstruction. Providing patients with comprehensive, unbiased information empowers them to make the choice that best aligns with their individual needs and goals for their reconstructed breasts and overall well-being.
Long-Term Safety Data on EVE and Similar Devices
Long-term follow-up data (often >10 years) provide reassurance:
* No Increased Cancer Incidence: Users show cancer rates consistent with general populations. No link found between the procedure and breast cancer.
* Sustained Tissue Health: Expanded tissue remains healthy and stable long-term.
* Low Rate of Serious Complications: Most issues are minor skin irritation. Serious complications like deep infection or significant tissue damage are rare with proper use. Patients can usually resume normal activities relatively quickly, sometimes advised to wear a supportive bra. Following instructions, perhaps including guidance on warm showers, is key.
This robust data supports external mechanical methods as a safe procedure without associated cancer development risk.
Parallel Applications: Learning from Orthopedics and Dentistry
Safe application of controlled mechanical forces extends beyond plastic surgery:
* Orthodontics: Gentle forces remodel bone/ligament to move teeth, used safely for generations.
* Distraction Osteogenesis: Orthopedic technique using tension to lengthen bones or regenerate defects (repair damage) with excellent long-term safety regarding malignancy.
* Craniofacial Surgery: Similar techniques expand skull/face bones safely.
Successful use across specialties involving different tissues (bone, ligament, skin, fat, muscle) underscores the principle that tissues adapt safely to appropriate mechanical stimuli, arguing against tension being inherently carcinogenic.
Beyond the Physical: Quality of Life and Non-Invasive Benefits
EVE's impact extends to well-being. For individuals needing reconstruction after trauma, burns, or cancer surgery, regaining wholeness is psychologically important for the reconstructed breast. External methods offer advantages:
* Non-Invasive Nature: Avoiding surgery reduces risks (anesthesia, infection, scarring, recovery). Beneficial for those not ideal surgical candidates or wishing to avoid more operations or implants.
* Autologous Tissue: Generates patient's own natural tissue (skin, fat) for optimal match (color, texture, sensation) at the recipient site.
* Empowerment: Active patient participation fosters control in recovery.
By safely restoring volume/contour without invasive surgery, EVE significantly contributes to improved body image, self-esteem, and quality of life, offering a valuable alternative in breast reconstruction pathways.
Future Directions in Mechanical Tissue Expansion
The field evolves, optimizing for outcomes, comfort, applications:
* Smart Devices: Sensors/feedback for real-time tension adjustments.
* Combination Therapies: Combining with growth factors/stem cells (with rigorous safety evaluation) possibly involving fat grafting.
* New Applications: Tissue engineering, organogenesis scaffolds.
* Material Science: Advanced biocompatible materials improve comfort.
Safety remains paramount as innovation refines these techniques, promising more effective, patient-friendly solutions compared to some surgery options involving extensive recovery.
Conclusion: Mechanical Expansion is Safe, Effective, and Life-Changing for Breast Reconstruction
Mechanical tissue generation, via devices like EVE, stands on solid science and clinical validation. Stimulating natural growth via controlled mechanical tension is biologically sound. Decades of research (studies, epidemiology, clinical follow-up) converge: mechanical forces, applied appropriately, do not cause cancer or increase cancer risk. This is crucial context for mastectomy patients considering reconstruction options.
Nipple reconstruction is also a crucial stage in the breast reconstruction process, typically taking place months after implant placement. The absence of mutagenic energy, confirmation from post-mastectomy reconstruction safety data (even with internal expanders), parallels with natural growth, and mechanotransduction pathways confirm oncologic safety. Misconceptions about triggering malignancy are unsupported. Comparing EVE to traditional breast reconstruction surgery highlights its non-invasive benefits and avoidance of implant-related complications like implant rupture or scar tissue issues, although each procedure has pros and cons best discussed with a surgeon or doctor.
For patients seeking reconstruction after trauma, cancer surgery, or exploring non-surgical aesthetic enhancement, external mechanical methods offer a pathway to healing, restoration, and quality of life that is effective and reassuringly safe, representing a triumph of biomedical engineering harnessing the body’s own regenerative potential for the breast. It’s a significant treatment option in the landscape of plastic surgery and reconstruction.
References
American Cancer Society: Breast Cancer Facts & Figures
FDA Medical Device Database: EVE Device Review
Journal of Plastic and Reconstructive Surgery: Tissue Expansion Safety Studies
PubMed Central: Mechanotransduction and Tissue Growth Research