Know These 6 Breast Implant Illness Symptoms
Share
Breast Implant Illness (BII) is a term used by patients and healthcare providers to describe a variety of symptoms that may be linked to breast implants. While BII is not yet a recognized medical diagnosis, increasing numbers of women report health issues they believe are connected to their breast implants. Understanding and identifying the symptoms of BII is crucial for seeking timely medical advice and intervention.
Here are six common symptoms associated with Breast Implant Illness.
1. Chronic Fatigue and Weakness
One of the most frequently reported symptoms of BII is chronic fatigue. Women with BII often experience symptoms that describe feeling persistently tired, even after a full night’s sleep. This fatigue can be debilitating, affecting daily activities and overall quality of life. Muscle weakness and a general lack of energy are also common, making it difficult to engage in physical activities or exercise.
2. Joint and Muscle Pain
Many women with BII experience significant joint and muscle pain. This pain can manifest as aching, stiffness, or swelling in various parts of the body. It often mimics conditions such as arthritis or fibromyalgia, making it challenging to diagnose. The joint pain can be widespread or localized to specific areas, such as the hands, knees, or back.
3. Cognitive Difficulties
Cognitive issues, sometimes referred to as "brain fog," are another common symptom of BII. Women report experiencing memory problems, difficulty concentrating, and confusion. These cognitive difficulties can impact work performance, social interactions, and overall mental well-being. The severity of these symptoms can vary, but they often lead to significant distress and frustration.
4. Autoimmune Responses
Breast implants can trigger autoimmune responses in some women. These responses may present as new autoimmune conditions or exacerbate existing ones. Common autoimmune symptoms of breast implant include rashes, hair loss, and thyroid problems. Some women develop conditions such as lupus, rheumatoid arthritis, or Sjogren’s syndrome after receiving breast implants.
5. Skin Rashes and Recurrent Infections
Skin rashes, ranging from mild irritation to severe dermatitis, are frequently reported by women with BII. These rashes can appear on the chest, around the implant area, or elsewhere on the body. Additionally, recurrent infections, including respiratory infections, urinary tract infections, and sinus infections, are common. These infections can be persistent and difficult to treat.
6. Breast Pain and Capsular Contracture
Breast pain and capsular contracture are direct symptoms related to the implants themselves. Capsular contracture happens when the scar tissue that naturally forms around the implant becomes tight and hard, leading to pain and discomfort.This condition can also alter the appearance of the breasts, making them look distorted or misshapen. Breast pain, whether due to capsular contracture or other factors, is a significant concern for many women with BII.
Determining the Cause of Your Symptoms
If you're experiencing unexplained symptoms or health problems, it's essential to consider that your breast implants might be the cause. Symptoms of Breast Implant Illness (BII) can mimic a variety of other illnesses, making it challenging to diagnose. Common symptoms include chronic fatigue, joint and muscle pain, cognitive difficulties, and autoimmune issues with such symptoms as rashes and hair loss. These symptoms are often attributed to other conditions, leading to a prolonged period of misdiagnosis and ongoing health issues.
According to the FDA, BII is not a formal medical diagnosis but rather a term used by patients and healthcare providers to describe a constellation of symptoms that appear to be related to breast implants. The variability of certain symptoms, and overlap of certain symptoms of these symptoms with other conditions make it imperative to seek medical advice from a professional experienced in BII.
Consulting with a plastic surgeon who is knowledgeable about BII is crucial for several reasons. First, an experienced surgeon can help rule out other potential causes of your symptoms through comprehensive medical evaluations and diagnostic tests. Second, they can provide detailed information about the risks associated with your specific type of breast implants, including whether they are silicone or saline, and whether they are textured or smooth.
In a study published in the journal Plastic and Reconstructive Surgery, researchers found that a multidisciplinary approach is often required to diagnose and manage BII. This approach may involve collaboration between plastic surgeons, rheumatologists, and other specialists to provide a thorough evaluation and effective treatment plan (Cohen Tervaert, J. W., & Kappel, R. M., 2013).
Furthermore, a plastic surgeon with expertise in BII can guide you through the process of implant removal if it is deemed necessary. Explantation (removal of breast implants) has been reported to lead to symptom improvement in many patients, though it is not guaranteed. Studies have shown that a significant number of women experience relief from symptoms following explantation, emphasizing the importance of seeking specialized care (Watad, A., Quaresma, M., Bragazzi, N. L., Cervera, R., & Tervaert, J. W. C., 2017).
It is also worth noting that ongoing research and advocacy efforts are continually improving our understanding of BII. Organizations like the American Society of Plastic Surgeons (ASPS) and the International Consortium of Investigative Journalists (ICIJ) provide valuable resources and updates on the latest findings related to breast implant safety and associated illnesses.
If you suspect that your breast implants may be causing your health problems, do not hesitate to seek professional medical advice. Early consultation with a qualified plastic surgeon can help determine the cause of your symptoms and set you on the path to recovery and improved well-being.
Recalled Breast Implants by the FDA
In recent years, the FDA has recalled certain breast implants due to potential health risks, including a rare form of cancer called Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL). The recall specifically targets textured implants and expanders manufactured by Allergan, which have been linked to a higher risk of this disease. The FDA advises patients with these implants to consult their healthcare providers for personalized medical advice and potential removal, emphasizing the importance of monitoring for any symptoms or complications.
Implant Complications
Breast implants, while generally safe, are not without potential complications. Being aware of these issues and discussing them with your surgeon is crucial to making informed decisions about your health and well-being. Here are some of the most common complications associated with breast implants:
Rupture: A rupture occurs when there is a tear or hole in the implant’s outer shell. This can happen in both saline and silicone implants, though the symptoms and consequences differ.
-
Saline Implant Rupture: When a saline implant ruptures, the saline solution leaks out and is absorbed by the body. The breast will typically deflate, leading to a noticeable change in shape and size. Saline ruptures are usually easy to detect due to this deflation.
-
Silicone Implant Rupture: Silicone implant ruptures are often referred to as "silent ruptures" because the silicone gel tends to remain within the surrounding scar tissue, making it less noticeable. Over time, however, the silicone can migrate and cause complications such as pain, swelling, and changes in breast shape. MRI or ultrasound screenings are recommended to detect silent ruptures.
Deflation: Deflation primarily affects saline implants and occurs when the saline solution leaks out due to a rupture. As mentioned, the breast will visibly deflate, which can be distressing and may require surgical intervention to replace or remove the implant.
Capsular Contracture: Capsular contracture is one of the most common complications following breast implant surgery. It occurs when the scar tissue (capsule) that naturally forms around the implant tightens and hardens. This can lead to:
-
Pain and Discomfort: The tightening of the capsule can cause significant pain and discomfort.
-
Changes in Breast Shape: The affected breast may appear misshapen or feel firm to the touch.
-
Treatment: Treatment options include medications, massage techniques, and in severe cases, surgical intervention to remove or replace the implant and the surrounding scar tissue.
Implant Displacement and Rotation: Implant displacement occurs when the breast implant moves from its original position. This can happen due to several reasons, including trauma, improper surgical technique, or natural changes in the body over time. Displacement can result in an unnatural breast appearance and may require surgical correction.
-
Rotation: Particularly with anatomically shaped implants, rotation can lead to an abnormal breast shape. Round implants are less affected by this issue, but it can still cause discomfort and aesthetic concerns.
Infection: Infection is a risk with any surgical procedure, including breast implant surgery. Infections of breast tissue can occur shortly after surgery or years later. Signs of infection include redness, swelling, fever, and pain. Treatment typically involves antibiotics, but in severe cases, the implant may need to be removed.
Seroma: A seroma is a buildup of fluid around the implant, which can cause swelling and discomfort. Seromas are more common shortly after surgery but can also develop later. They are usually managed by draining the fluid with a needle, though persistent seromas may require surgical intervention.
What is Silicone Toxicity?
Silicone toxicity refers to the adverse health effects that can occur when silicone, particularly from silicone gel-filled breast implants, leaks or ruptures and migrates into surrounding tissues or the bloodstream. While the silicone gel filled implants is generally considered biocompatible, meaning silicone breast implants are usually well-tolerated by the body, there are instances where the immune system reacts adversely, leading to a range of symptoms and other health concerns and issues.
When Do Breast Implant Illness Symptoms Start?
BII symptoms can start at any time after getting implants, even years later. It’s important to monitor your health and note any changes that could be linked to your implants.
Immediate vs. Delayed Onset
The onset of BII symptoms can be categorized into immediate and delayed reactions:
-
Immediate Onset: Some women experience symptoms shortly after surgery, within days or months. These early symptoms might be related to the body’s initial response to the implant, such as inflammation, infection, or acute allergic reactions. Early-onset symptoms can include pain, swelling, and redness around the implant site.
-
Delayed Onset: In many cases, symptoms of BII do not appear until years after the implants have been placed. This delayed onset is often due to the body’s gradual immune response to the implants or the slow leakage of silicone in the case of silicone gel-filled implants. Symptoms such as chronic fatigue, joint pain, and cognitive issues may develop gradually, making it harder to immediately associate them with the breast implants.
Factors Influencing Symptom Onset
Several factors can influence when BII symptoms start:
-
Type of Implant: The material and type of breast implant (saline vs. silicone, smooth vs. textured) can impact the onset of symptoms. Silicone implants, especially if they rupture or leak, may cause a delayed immune response that leads to symptoms appearing years after surgery.
-
Individual Immune Response: Each person’s immune system reacts differently to foreign objects in the body. Some women may have a more immediate and pronounced reaction, while others may have a delayed response.
-
Capsular Contracture: The development of capsular contracture, where scar tissue forms around the implant and tightens, can contribute to the onset of symptoms. This condition can occur months or years after the initial surgery and may cause pain and changes in breast appearance.
-
External Factors: Lifestyle, environmental exposures, and other health conditions can also influence when and how symptoms appear. For example, stress, illness, or hormonal changes might trigger or exacerbate BII symptoms.
Importance of Regular Monitoring
Given the unpredictable nature systemic symptoms of BII, regular monitoring and proactive health checks are crucial. Women with breast implants should:
-
Keep Detailed Health Records: Document any new or unusual symptoms, including their onset, duration, and severity. Keeping a health diary can help identify patterns and potential triggers.
-
Regular Medical Check-Ups: Schedule regular check-ups with your healthcare provider, and discuss any concerns or symptoms. Imaging studies, such as MRIs or ultrasounds, can help detect issues like implant rupture.
-
Stay Informed: Keep up to date with the latest research and guidelines on breast implants and BII. Awareness of potential risks and symptoms can empower you to seek timely medical advice.
-
Consult Specialists: If symptoms persist or worsen, consider consulting a specialist experienced in diagnosing and treating BII. A multidisciplinary approach involving rheumatologists, endocrinologists, and plastic surgeons may be necessary for comprehensive care.
Research and Advocacy
Ongoing research and advocacy efforts are critical in understanding BII and improving patient care. Studies aim to uncover the underlying mechanisms of BII, identify risk factors, and develop effective treatments. Organizations and patient advocacy groups are working to raise awareness and support women experiencing BII.
Breast Implants and Cancer Risk
While rare, there is a risk of developing Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) and other cancers in women with breast implants. BIA-ALCL is a type of non-Hodgkin's lymphoma that can develop in the scar tissue and fluid surrounding the implant. Regular check-ups and monitoring are crucial for early detection and treatment of this and other potential implant-related complications. Being vigilant about changes in breast appearance, new lumps, or persistent swelling can aid in early diagnosis and improve outcomes.
Additionally, some studies suggest a possible link between breast implants and a higher incidence of other types of cancer, although the evidence is not conclusive. This potential risk underscores the importance of ongoing research and patient education. Women with breast implants should maintain regular communication with their healthcare providers, stay informed about the latest findings, and participate in recommended screening programs to ensure any issues are detected and addressed promptly.
Breast Implants and Breast Cancer
It is essential to understand that while breast implants do not cause breast cancer, they can complicate the detection of cancerous growths. Implants can obscure mammogram images, making it harder to detect tumors early. Specialized imaging techniques, such as implant displacement views and MRIs, are often recommended for women with implants to ensure thorough screening. Awareness of this issue and working closely with healthcare providers can help mitigate the risk and ensure that breast cancer, if it develops, is caught at an early, more treatable stage.
Breast Implants and Connective Tissue Disease (CTD)
There is ongoing debate about the link between breast implants and connective tissue disease (CTD). Some studies suggest a possible association, while others do not, leading to conflicting opinions within the medical community. Women with a serious personal or family history of autoimmune diseases, such as lupus, rheumatoid arthritis, or scleroderma, should have a thorough discussion with their surgeon about these potential risks. It's important to weigh the benefits and risks of breast implants carefully and consider alternative options if there are significant concerns about triggering or exacerbating autoimmune conditions.
Recent research has attempted to clarify this potential connection, but results remain inconclusive. The variability in study designs, patient populations, and types of implants used contribute to the ongoing uncertainty. Despite the lack of definitive evidence, some patients with pre-existing autoimmune conditions report symptom exacerbation after receiving breast implants. As a result, a personalized approach to risk assessment and decision-making is crucial. Healthcare providers must stay informed about the latest research and guidelines to offer the best advice and support to their patients.
Dr. Khouri’s Method of Breast Implant Removal
Education is key for patients considering breast implant removal. Understanding the procedure, potential outcomes, and recovery process can help you make an informed decision. Whether you're experiencing complications, such as Breast Implant Illness (BII), or simply wish to remove your implants for personal reasons, being well-informed is crucial for achieving the best possible outcome.
The Decision to Remove Breast Implants
The decision to remove breast implants can stem from various reasons:
-
Health Concerns: Issues such as chronic pain, autoimmune reactions, or symptoms associated with BII may prompt the need for explantation.
-
Aesthetic Preferences: Changes in body image or personal preferences over time might lead someone to opt for implant removal.
-
Complications: Implant-related problems, such as rupture, capsular contracture, or infection, often necessitate removal.
Understanding Dr. Khouri’s Explantation Procedure
Dr. Khouri’s explantation procedure involves replacing implants with the patient’s own fat through a fat transfer technique. This method addresses common implant problems, avoiding future re-operations and ensuring breast reconstruction has a natural feel and appearance.
What to Expect:
-
Consultation: Your surgeon will discuss your medical history, reasons for explantation, and desired outcomes. This is also an opportunity to address any concerns and ask questions.
-
Surgery: The procedure typically involves removing the implants and transferring fat to fill the stretched tissue. Additional procedures like a breast lift may be performed to improve the breast’s appearance post-removal.
-
Recovery: Post-surgery, patients can expect a recovery period involving rest, limited physical activity, and follow-up visits with the surgeon. Full recovery can take several weeks to months, during which the body heals and adjusts to the changes.
Potential Outcomes and Recovery Process
The outcomes of explantation can vary based on individual circumstances and the specifics of the plastic surgery itself:
-
Immediate Relief: Many patients report immediate relief from symptoms associated with BII or other implant-related issues.
-
Aesthetic Changes: The breasts may look different after implant removal. Dr. Khouri’s fat transfer technique helps achieve a more natural appearance.
-
Emotional Well-being: Improved physical health often leads to better emotional well-being, as patients feel more comfortable and confident in their bodies.
Patient Example:
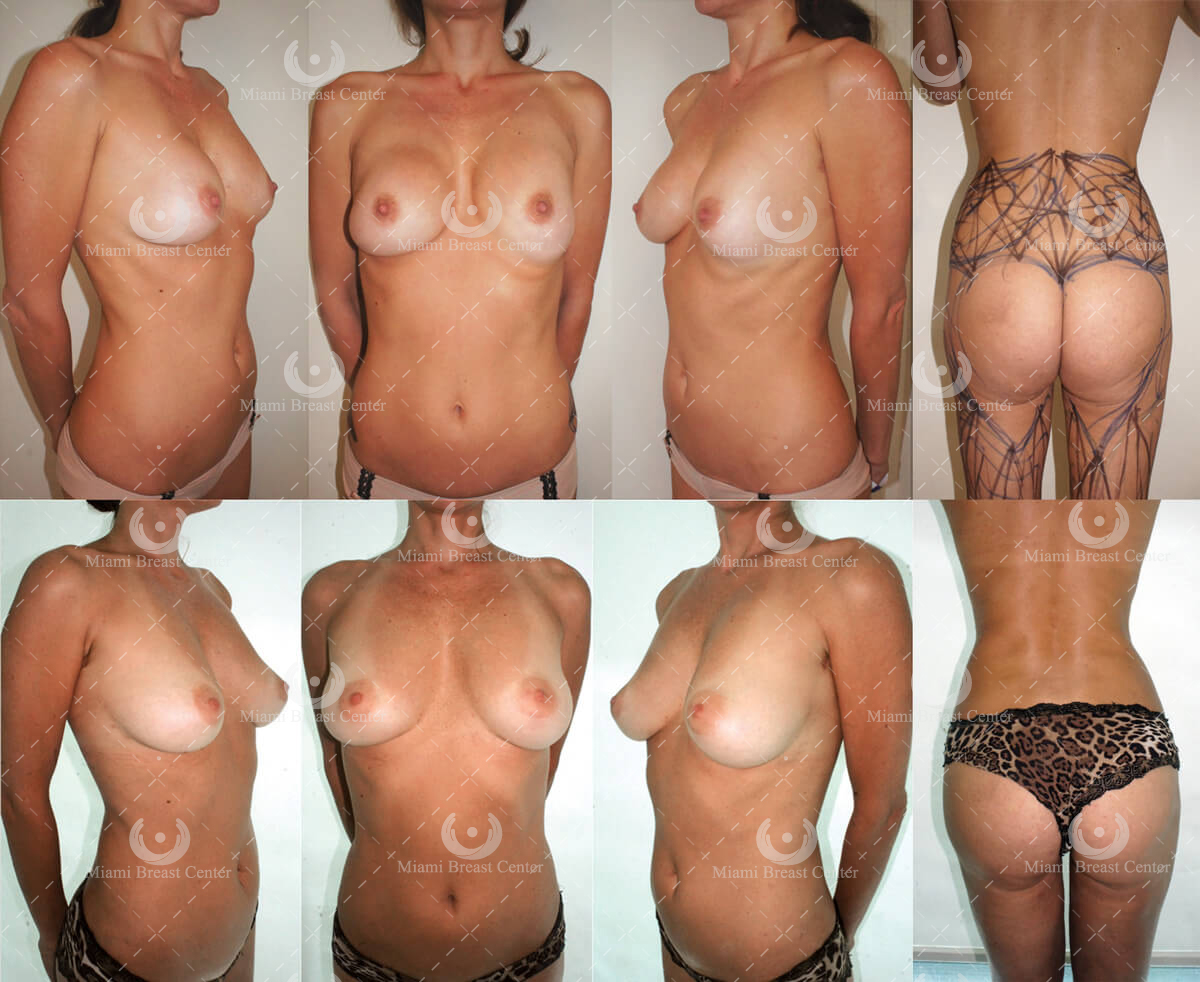
"Thirty-nine-year-old woman who had cosmetic breast augmentation with silicone implants 10 years prior to her presentation. Plastic surgeons insert implants either under the muscle or over the muscle. In her case, she had the right breast implant put over the muscle that developed a deforming and painful capsular contracture and the left implant under the muscle that migrated inferiorly resulting in loss of the normal breast fold.
We removed her implants and restored breast volume with microfat grafts meticulously placed in the tissue plane between the implant capsule and the skin. The capsule was treated with PALF and the breast fold was restored with a RAFT.
She is shown 1-year post-grafting implant free with satisfactory breast shape and volume. Note that even in the skinniest of patients we can always harvest enough fat from the “love handles” and give the patient an improved waistline contour (far right)."
Source: https://www.miamibreastcenter.com/implants/removal
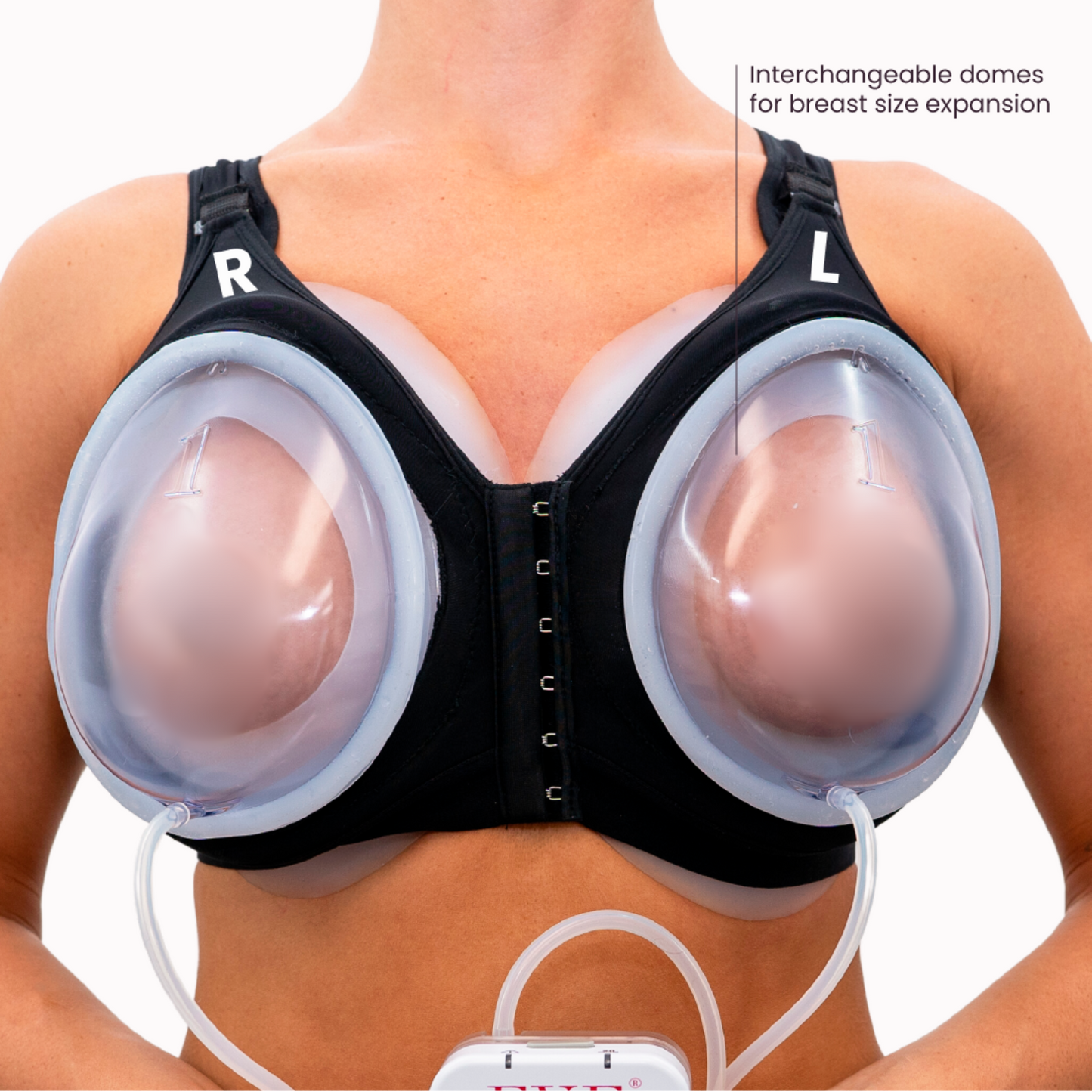
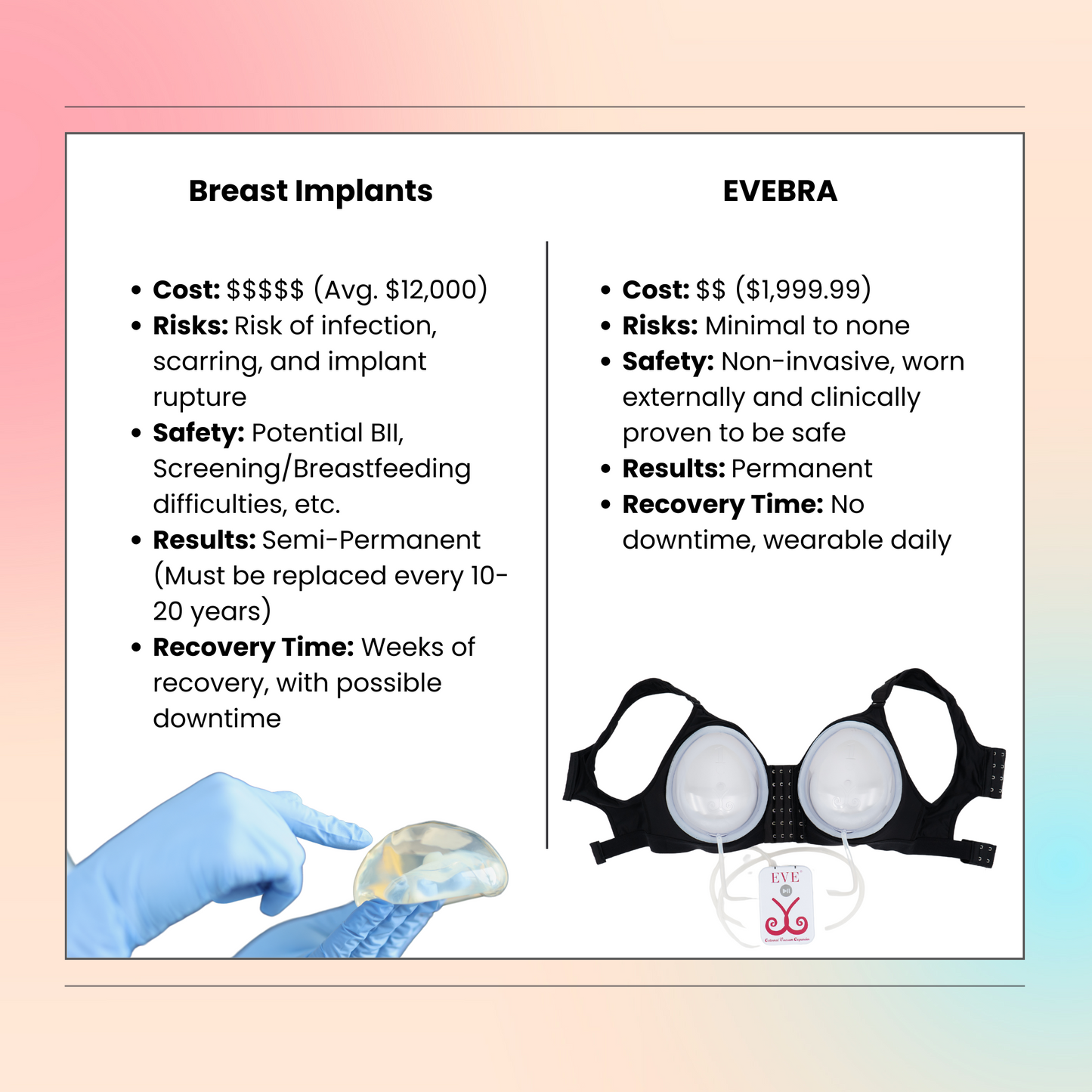
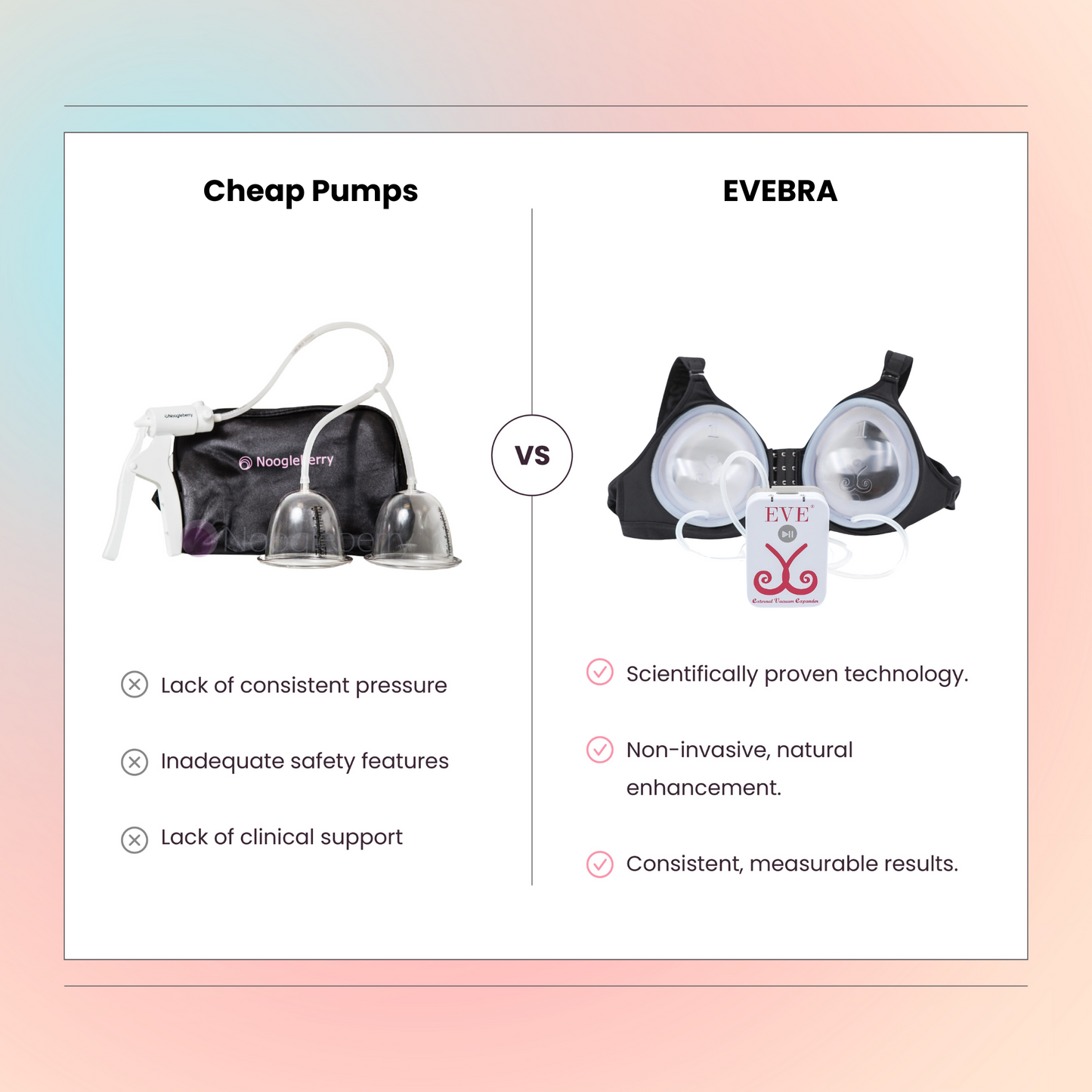
Exploring Non-Surgical Breast Enlargement: Introducing EVEBRA
For patients looking to maintain or enhance breast volume after explantation without undergoing additional surgeries, EVEBRA offers an innovative solution. EVEBRA is a non-surgical breast enlargement device designed to provide a safe, effective, and natural alternative to surgical procedures.
How EVEBRA Works
EVEBRA utilizes advanced technology to promote natural breast tissue growth. Here’s how patients can benefit from EVEBRA after explantation:
-
Non-Invasive Approach: Unlike surgical procedures, EVEBRA is a non-invasive device that stimulates breast tissue growth without incisions or recovery time.
-
Gradual Enhancement: The device encourages gradual, natural enlargement of the breasts over time, helping to restore volume and shape.
-
Safe and Effective: EVEBRA is designed with safety in mind, providing a reliable alternative for breast enhancement without the risks associated with surgery.
Using EVEBRA After Explantation
Patients who have undergone explantation can integrate EVEBRA into their post-surgery recovery and enhancement routine:
-
Consultation: Before starting EVEBRA, consult with your healthcare provider to ensure it’s suitable for your individual health and recovery status.
-
Regular Use: Follow the recommended usage guidelines for EVEBRA to achieve the best results. Consistent use as directed can lead to noticeable improvements in breast volume and shape.
-
Monitoring Progress: Regularly monitor your progress and maintain follow-up appointments with your healthcare provider to ensure optimal results and address any concerns.
Benefits of EVEBRA for Post-Explantation Enhancement
EVEBRA offers several benefits for patients seeking non-surgical breast enlargement after explantation:
-
Natural Look and Feel: EVEBRA promotes natural tissue growth, resulting in a more natural look and feel compared to synthetic implants.
-
Minimized Risk: As a non-invasive option, EVEBRA reduces the risk of complications associated with surgical procedures, such as infections or scarring.
-
Convenience and Comfort: EVEBRA is designed for ease of use, allowing patients to incorporate it into their daily routines without significant lifestyle disruptions.
Conclusion
Understanding Breast Implant Illness (BII) and its symptoms is crucial for women who have breast implants. Although BII is not yet a recognized medical diagnosis, the reported symptoms such as chronic fatigue, joint and muscle pain, cognitive difficulties, autoimmune responses, skin rashes, and breast pain highlight the need for awareness and timely medical consultation. If you suspect that your breast implants might be causing health issues, seek advice from a healthcare provider experienced in BII. Early diagnosis and appropriate intervention can significantly improve your health and well-being.
Moreover, the FDA's recall of certain breast implants due to the risk of Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) underscores the importance of vigilance and regular monitoring for those with implants. Staying informed about potential risks and emerging research can empower patients to make proactive decisions about their health. Consulting with specialists and considering alternatives like EVEBRA for non-surgical breast enlargement can provide safer, effective solutions for those affected by implant-related complications.
In addition, it is essential to foster a supportive community for women experiencing BII. Sharing experiences, resources, and knowledge can help raise awareness and drive further research into the causes and treatments of BII. Advocacy groups and medical professionals working together can play a pivotal role in improving patient outcomes and advancing our understanding of this complex condition. By staying informed and engaged, women can navigate their health journeys with confidence and support.
References:
-
Cohen Tervaert, J. W., & Kappel, R. M. (2013). Silicone implant incompatibility syndrome (SIIS): A frequent cause of ASIA (Shoenfeld's syndrome). Immunologic Research, 56(2-3), 293-298.
-
Watad, A., Quaresma, M., Bragazzi, N. L., Cervera, R., & Tervaert, J. W. C. (2017). The autoimmune/inflammatory syndrome induced by adjuvants (ASIA) syndrome as an explanatory model of autoimmunity after silicone implants: a systematic review of the literature. Lupus, 26(6), 675-682.
-
U.S. Food and Drug Administration (FDA). (2020). Breast Implants - Certain Labeling Recommendations to Improve Patient Communication. Link.
-
U.S. Food and Drug Administration (FDA). (2020). Breast Implants - Certain Labeling Recommendations to Improve Patient Communication. FDA Website.
-
U.S. Food and Drug Administration (FDA). (2019). FDA Takes Action to Protect Patients from Risk of Breast Implants. FDA News Release.
-
American Society of Plastic Surgeons (ASPS). (2020). FDA Announces New Breast Implant Safety Measures. ASPS Website.
-
McCarthy, C. M., Cano, S. J., Klassen, A. F., Scott, A. M., & Pusic, A. L. (2012). The magnitude and timing of changes in health-related quality of life following aesthetic breast augmentation: A meta-analysis of prospective studies. Plastic and Reconstructive Surgery, 130(4), 788-792.
-
U.S. Food and Drug Administration (FDA). (2021). Breast Implant Rupture. FDA Website.
-
American Society of Plastic Surgeons (ASPS). (2021). Saline Breast Implants. ASPS Website.
-
Plastic and Reconstructive Surgery Journal. (2017). Management of Capsular Contracture in Breast Augmentation: A Comprehensive Review of the Literature. Journal Article.
-
Mayo Clinic. (2020). Breast Implants: Complications. Mayo Clinic Website.
-
Journal of Clinical and Aesthetic Dermatology. (2016). Management of Post-Surgical Infections in Breast Augmentation. Journal Article.
-
American Journal of Surgery. (2019). Management of Seroma in Breast Surgery. Journal Article.
-
American Society of Plastic Surgeons (ASPS). (2020). Breast Implant Illness (BII) – Symptoms, Risks, and Safety. ASPS Website.
-
U.S. Food and Drug Administration (FDA). (2021). Breast Implants – Safety Information. FDA Website.
-
National Center for Biotechnology Information (NCBI). (2018). Breast Implant Illness: A Review. NCBI Website.